What’s Diosgenin ? Why was Diosgenin selected ?
Diosgenin is classified in the group of steroid sapogenin (The chemical structure of diosgenin is same as the steroid hormons.), and it is known as component of the root of Dioscorea japonica and Dioscorea batatas .
Diosgenin is contained in herbs such as Trigonella spp., Polygonatum spp., Smilax spp. and other several plants.
Diosgenin has the diversified pharmacological activities, and the following pharmacological activities are reported, based on the animal model experiments ;
(1) anticancer activity
(2) alleviating activity against food allergy
(3) Inhibitory effect for memory impairment induced by oxidative stress through administration of galactose
(4) Improvement effect for diabetic neuropathy
Safety of Diosgenin
The safety of Diosgenin through oral intake is clearly described in the Natural Medicines Comprehensive Database, which is the authorized database for supplements by FDA, NIH and other many countries’ health authorities. And Diosgenin showed no acute toxicity even at 6000mg/kg per oral administration in rats and mice, which proves that diosgenin is the safe compound.
Effect of Diosgenin on Brain Function
Based on a series of researches performed by Professor C.Tohda, it was made clear that Diosgenin could improve the dementia and the cognitive functions as follows.
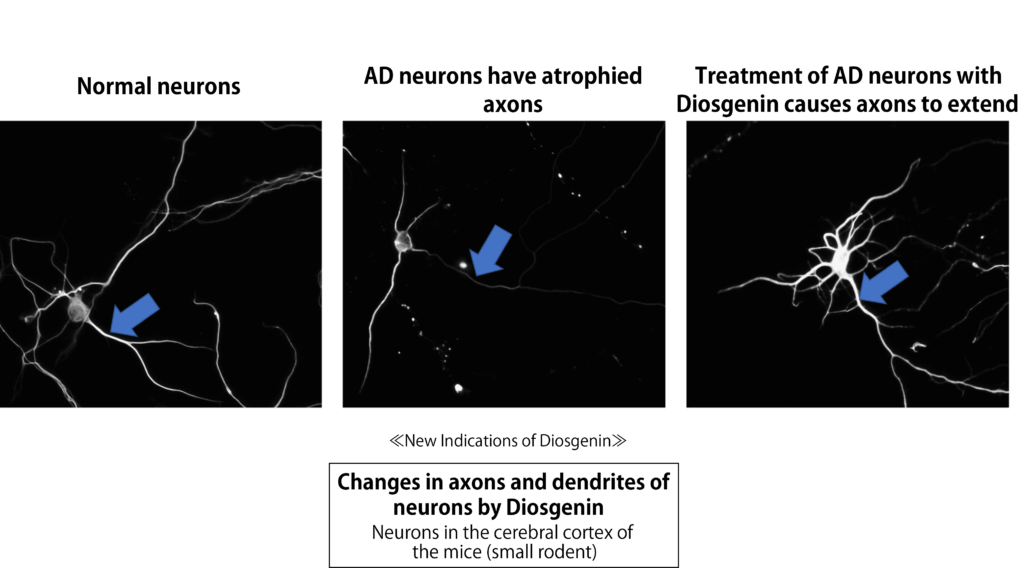
When the cultured neuron was treated with amyloid β which causes Alzheimer disease, the axons and dendrites of neurons were both atrophied. Surprisingly, the atrophied axons and dendrites of neuron became resilient and extended thereafter it was treated with Diosgenin (5).
Furthermore, the improvement activity of Diosgenin to Alzheimer disease was confirmed through the experimental researches with Alzheimer-model 5XFAD mice (6).
5XFAD mice are the transgenic mice which express human APP and PSEN1 transgenes with a total of five family-related AD-linked mutations: the Swedish (K670N/M671L), Florida (I716V), and London (V717I) mutations in APP, and the M146L and L286V mutations in PSEN1. The deposition of Amyloid β is observed in the brain at 2 months old, and the synaptophysin-positive presynapses starts decreasing in the brain of more than 4 months old in 5XFAD mice.
In regards to memory impairment, the short-term working memory is damaged at 4 – 5 months of age, and object recognition memory, special memory and fear memory are damaged at 5 – 6 months of age in 5XFAD mice, respectively. After administration of Diosgenin to 6 – 8 months old 5XFAD mice with advanced memory impairment, the memory impairment was significantly improved up to the same level as the wild- type mice (5). The axonal terminal ovoids and the swollen presynapses indicating axonal denaturation are observed at the same site as amyloid β plaque deposition mainly in the cerebral cortex and hippocampus. Neurofibrillary tangel due to excess tau phosphorylation is also clearly observed in 5XFAD mice. These all characteristic features of the Alzheimer model 5XFAD mice were significantly reduced by Diosgenin treatment.
In addition, it was also found that Diosgenin administration to normal mice facilitated memorizing ability. By diosgenin treatment, spike firing frequencies were enhanced and the axon density was increased in the medial prefrontal cortex and hippocampus which were important areas for memory formation. Thus, it was indicated that Diosgenin had the activity to improve the cognitive functions not only in Alzheimer disease but also even in healthy state.
Action Mechanism of Diosgenin
Several scientific articles has been reported with the action mechanism of Diosgenin except the neuroscience research area , such as the inhibition of phosphorylation for TNF-α induced Akt, ERK, JNK, p38 in the vascular smooth muscle cell(8)and the inhibition of phosphorylation for STAT3 in the liver cancer cell(9).
On the other hand, the mode of action of Diosgenin in the nervous system had not been clarified yet until Professor C.Tohda made it clear. She has implemented the DARTS(drug affinity responsive target stability) analysis to brain nerve system in order to clarify the target protein of Diosgenin, And finally she found Diosgenin bound to the target protein 1,25D3-MARRS(1,25D3-membrane-associated rapid-response steroid binding protein) in brain nerve system,which resulted in playing the pharmacological actions(5).
1,25D3-MARRS was identified as the receptor for inducing the rapid response of active form VD3 without transcription(11), but the function in the nerve system had not been reported.
Diosgenin interacts with 1,25D3-MARRS on the cell surface of neurons, and activates at least PKA, PKC, ERK, PI3K in the downstream. Furthermore, it was found that the resulting decrease of intracellular heat shock cognate 70 (HSC70) was the important event to induce the axon extension and memory improvement(12,13). 1,25D3-MARRS is the protein expressing in not only mice but also human.
The Clinical Effect in Healthy Volunteers
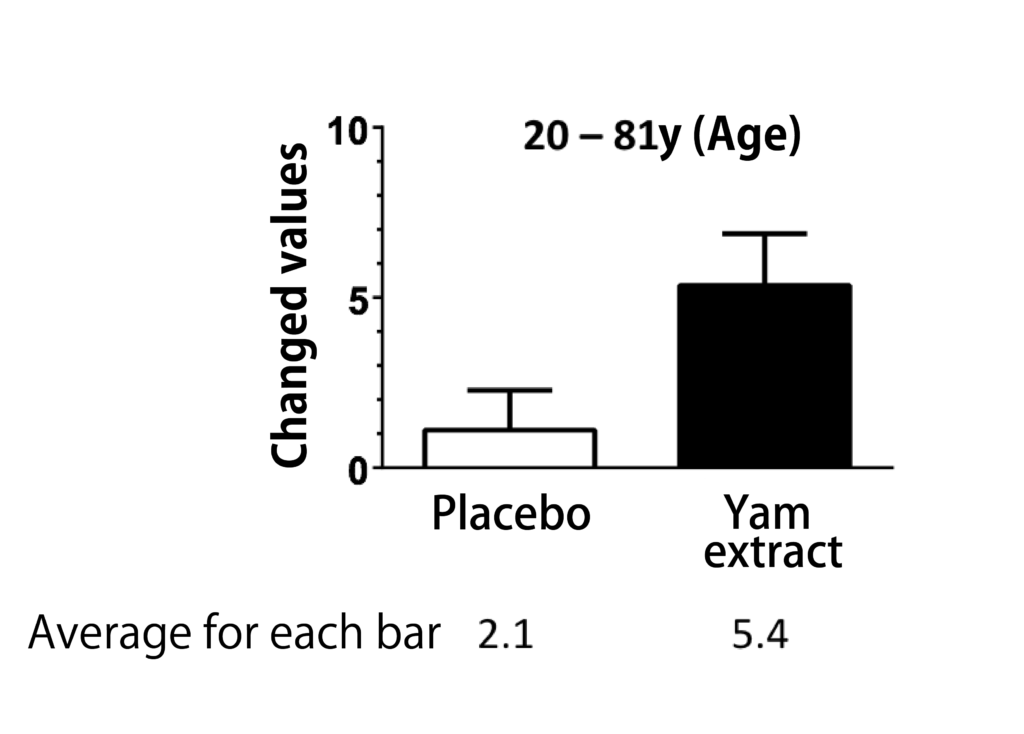
The project team led by Professor C.Tohda performed the clinical research to investigate the cognitive function improvement action with the same supplement soft capsule formulation as Diosgenin Gold containing Diosgenin-rich Yam extract in healthy volunteers.
The clinical research trial was designed in randomized placebo-controlled double-blinded crossover study, which is one of the most strict clinical trial designs, and the RBANS test was used as evaluation method on cognitive function improvement level.
In the clinical research trial, it was clearly confirmed that the clinical trial sample, soft capsule containing Diosgenin-rich Yam extract, showed the effectiveness statistically to improve the cognitive function against the placebo(14), and no side effects were observed by taking the soft capsule containing Diosgenin-rich Yam extract.
Reference
1) Yan LL, et al. Exp Oncol. 2009;31:27-32.
2) Huang CH, et al. Planta Med. 2009:75:1300-1305.
3) Chiu CS, et al. Am J Chin Med. 2011:39:551-563.
4) Kang TH, et al. Biol Pharm Bull. 2011:34:1493-1498.
5) Tohda C, et al. Sci Rep. 2012;2:535.
6) Oakley H, et al. J Neurosci. 2006;26:10129-10140.
7) Tohda C, et al. Sci Rep. 2013;3:3395.
8) Choi K, et al. Vascul Pharmacol. 2010;53:273-280.
9) Li F, et al. Cancer Lett. 2010;292:197-207.
10) Lomenick B, et al. Proc Natl Acad Sci USA. 2009;106:21984-21989.
11)Nemere I, et al. Proc Natl Acad Sci USA. 2004;101:7392-7397.
12)Yang X and Tohda C. Front Pharmacol. 2018;9:48.
13)Yang X and Tohda C. Sci Rep. 2018;8:11707.
14)Tohda C, et al. Nutrients 2017; 9:1160.