Introduction.
It is estimated that 4.62 million people in Japan have dementia and 4 million people have mild cognitive impairment (as of 2012, according to the Ministry of Health, Labour and Welfare), and the number is increasing every year. Alzheimer’s disease accounts for the majority of the causes of dementia. The number of people with Alzheimer’s disease is high worldwide, and the number is increasing every year, making it a major social problem (1).
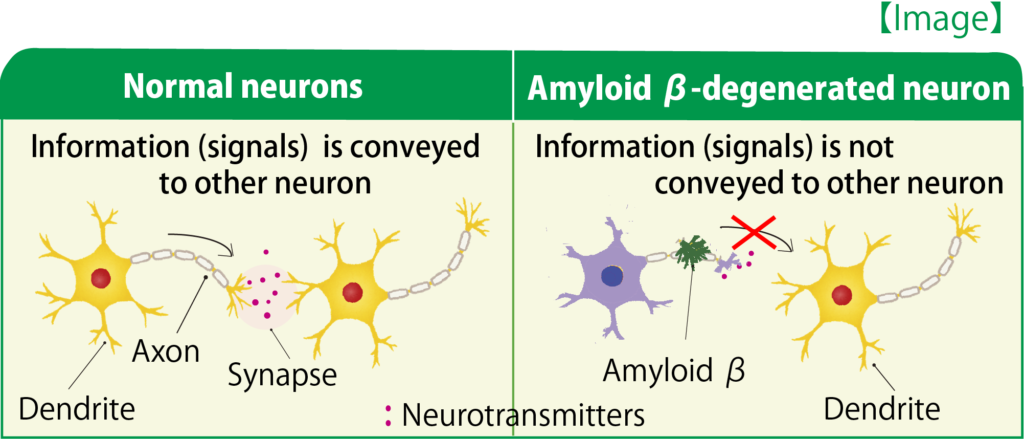
Alzheimer’s disease is a progressive neurodegenerative disease characterized by amyloid beta (Aβ) deposition in the brain and neurofibrillary changes (axonal accumulation of excess phosphorylated tau aggregates). Core symptoms include generalized impairment of cognitive functions such as memory impairment, disorientation, impaired comprehension and judgment, executive dysfunction, language disorder (aphasia), and aphasia, while peripheral symptoms include irritability, agitation, agitation and excitement, de-inhibition, abnormal behavior, delusions, hallucinations, depression, anxiety, euphoria, apathy, abnormal nocturnal behavior, and abnormal eating behavior. It shows. Aβ accumulation and neuronal degeneration have been shown to begin approximately 20 years prior to the diagnosis of Alzheimer’s disease (2-6).
Mild cognitive impairment (MCI) is described as “cognitive decline (reported by the individual or a third party or impaired by objective cognitive tests), but basic daily activities are maintained and are not dementia” (2-6). However, mild cognitive impairment can progress to Alzheimer’s disease, as a meta-analysis reported that 32% of these individuals will progress to Alzheimer’s disease within 5 years (7), but there is no drug therapy for MCI.
The three drugs approved for Alzheimer’s disease are the cholinesterase inhibitors donepezil, rivastigmine, and galantamine, and the NMDA receptor antagonist memantine. Cholinesterase inhibitors were developed for the treatment of Alzheimer’s disease because it was recognized at the time of their development that the cause of Alzheimer’s disease was a lack of acetylcholine in the brain.
However, subsequent research has shown that Alzheimer’s disease causes extensive neuronal degeneration due to the accumulation of Aβ, and cholinergic nerve shedding is part of the result. In the absence of acetylcholine-releasing axon terminals projecting to target neurons (i.e., axons and dendrites are degenerating and atrophying), cholinesterase inhibitors are not expected to work. The widespread neurodegeneration in the brain also reduces axonal projection of glutamatergic neurons. The same is true in this condition, where administration of the NMDA receptor antagonist memantine is unlikely to be effective. In other words, standard treatments are symptomatic and their effects become more difficult to exert as the neurodegeneration progresses.
In looking at the development of new therapies with different mechanisms of action than those already approved, there are many candidates that have reached clinical trials, but so far none have been approved. For example, neither solanezumab (8), an antibody against Aβ, nor verubecestat (9), a beta-secretase inhibitor, showed cognitive benefit in Phase 3 trials. A candidate drug designed to inhibit neurofibrillary changes also failed to improve cognition in a Phase 3 study (10).
By the time the diagnosis of Alzheimer’s disease is made, systematic degeneration has progressed, and even if the cause of the disease is reduced, recovery of brain function will not be achieved, because the accumulation of Aβ in the brain has already begun during the asymptomatic phase of the disease, which dates back 20-30 years before the Alzheimer’s stage. Therefore, our research has been based on the idea that “stopping or even repairing the disruption of the neural network is critically important for the maintenance and recovery of cognitive function”.
1.What is Diosgenin?
Diosgenin is a steroidal sapogenin compound (Fig. 1) and is a component of mountain medicines (Dioscorea japonica or the rhizome of D. batatas), which are used in Chinese medicine for nourishing and tonic effects. In addition, other species of the genus Dioscorea, Trigonella spp. and Polygonatum spp. and Smilax spp. also contain diosgenin. In the so-called food poisoning category, wild medicines are classified as “component essences that are not judged as medicines unless they have a medicinal effect” and can be used as health food materials.
2.Effects of Diosgenin in an Alzheimer’s disease model (11)
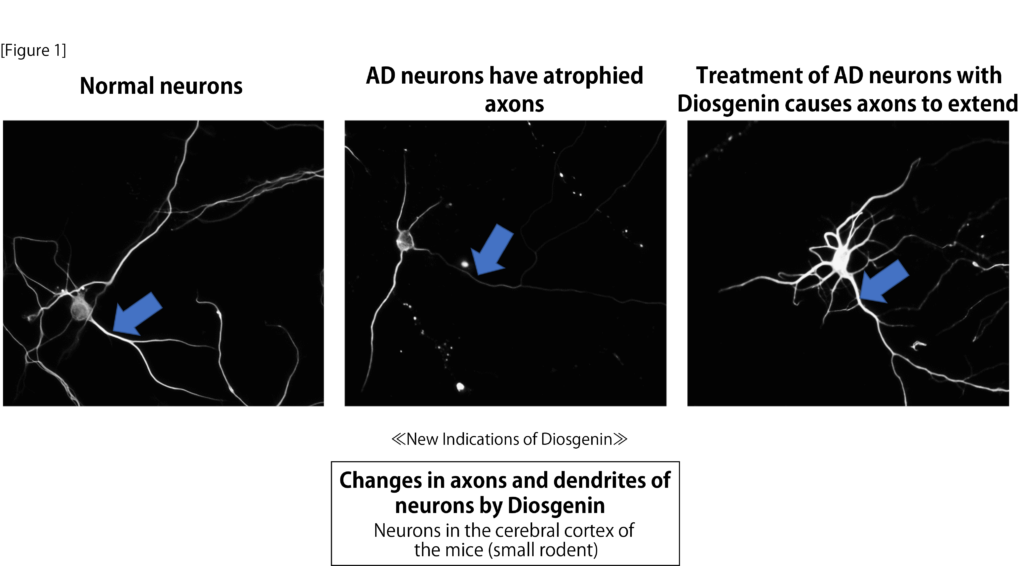
We first performed experiments in cultured neurons that mimicked the rupture and degeneration of the neural network in the Alzheimer’s disease brain. After treatment of the neurons with Aβ, axons and dendrites were atrophied, and then treated with diosgenin, the neurons showed a pronounced re-extension of the neurons. We next examined the anti-Alzheimer’s effects of diosgenin in the 5XFAD mouse model of Alzheimer’s disease: at 2 months of age, Aβ was deposited in the brains of the 5XFAD mice, presynaptic loss began at 4 months of age or later, short-term working memory was observed at 4-5 months of age, and at 5-6 months of age, diosgenin was found to have a significant effect on This is a model in which object cognitive, spatial, and fear memories are impaired in 5XFAD. We administered diosgenin intraperitoneally for 20 days to 6-8 month old 5XFAD mice, which were significantly impaired, and their memory deficits improved to the same extent as in wild-type mice. In addition, the 5XFAD mice had spherical axonal endings and presynaptic hypertrophy, which are images of axonal degeneration, overlying Aβ plaques in the cortex and hippocampus, as well as significant neurofibrillary changes (tau hyperphosphorylation), which were significantly reduced in the diosgenin group.
Diosgenin improves memory deficits not only by intraperitoneal administration, but also by oral administration (12). However, the drug had to be dissolved in a special solvent to be effective orally. Without this appropriate solvent, the amount of diosgenin transferred to the brain was extremely low and no improvement in memory impairment was observed (13), and a patent was obtained for a series of findings (inventor: Chihiro Tohda).
3.Diosgenin’s effect on memory in normal mice (14)
Diosgenin also enhanced memory performance in normal mice, with a significant increase in the frequency of neuronal firing in the frontal cortex and hippocampus, as well as an increase in axonal density in those areas. The results suggest that Diosgenin has a positive effect on the body.
4.Analysis of the mechanism of action of Diosgenin (11,14)
A comprehensive analysis using the drug affinity responsive target stability (DARTS) method (15) to identify the direct target molecule of diosgenin revealed that the proteins that bind to diosgenin are 1,25D3-membrane associated rapid-response steroid binding protein (1,25D3-MARRS). 1,25D3-MARRS has been shown to be a receptor for vitamin D3 in vivo, but its function in the nervous system has not been reported. Our study highlighted for the first time a molecule involved in axon stretching and memory improvement (Figure 1). Furthermore, we examined the changes that occur in neurons after stimulation of 1,25D3-MARRS by diosgenin and found that a decrease in heat shock cognate 70 was involved (12,16). 5.
5.Effects of Diosgenin in humans (13)
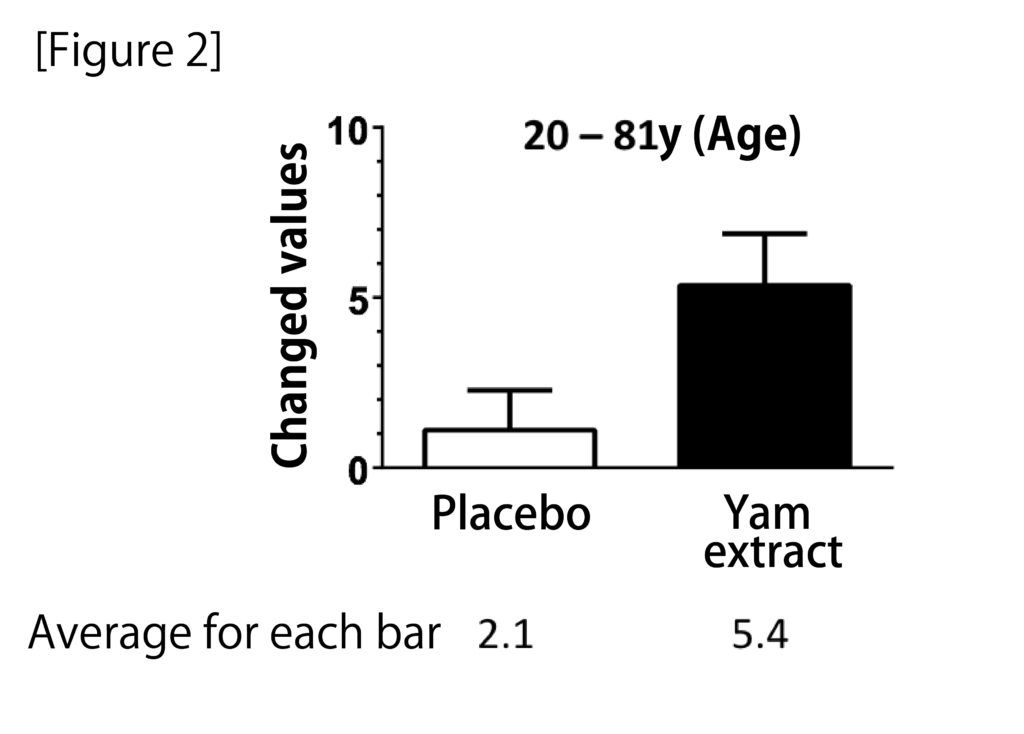
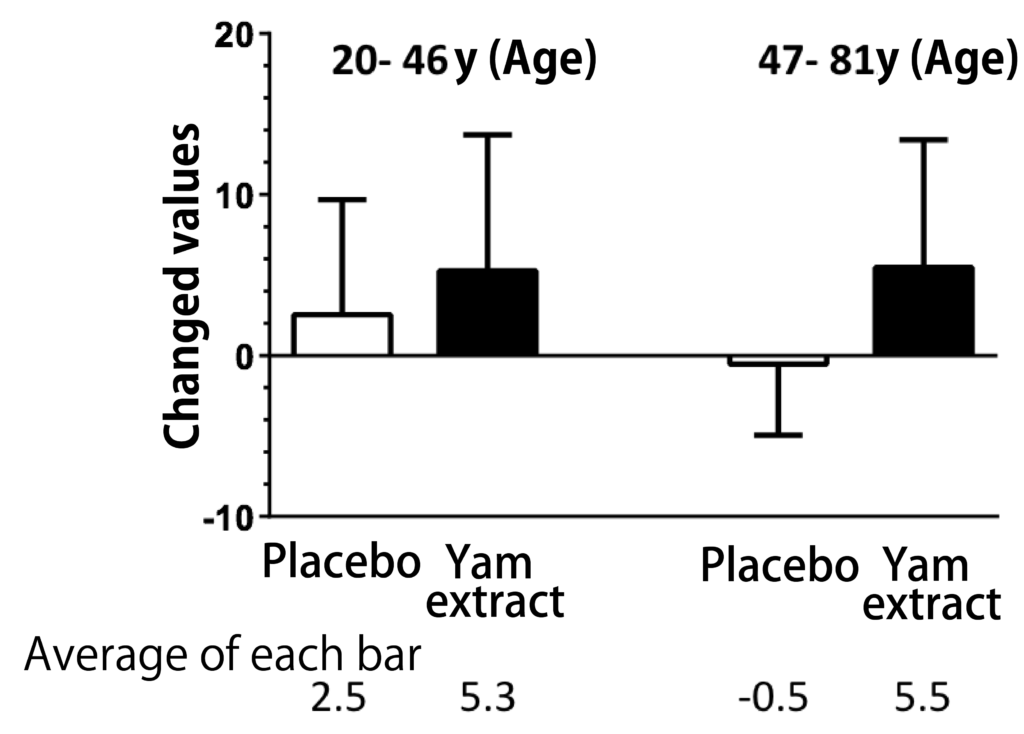
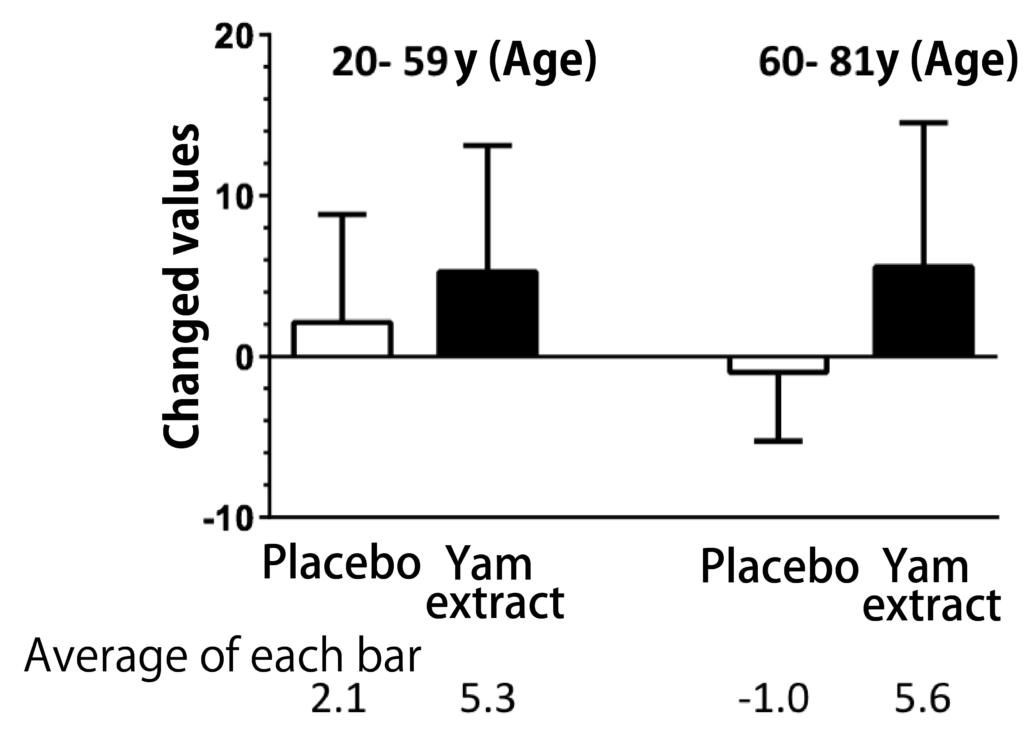
A randomized, double-blind, crossover study was conducted with 28 participants aged 20 to 81 years to determine the cognitive function of the diosgenin-containing yam extracts in healthy subjects in the Repeatable Battery for the It was assessed by the Assessment of Neuropsychological Status (RBANS) test. Taking yam extract for 3 months significantly improved the total RBANS scores, and when analyzed for each of the five cognitive function domains by age, there was an improvement in delayed memory, especially at age 60 years and older (Figure 2). These results suggest that yam extract containing diosgenin activates cognitive functions such as memory ability.
Conclusion.
As described above, diosgenin, which has the ability to strengthen and repair the connections between nerve cells in the brain, was found to improve Alzheimer’s disease and enhance normal memory in animal studies. It is important to note that diosgenin and diosgenin-containing yam extracts, when consumed as-is (or eaten) or administered orally in a water-soluble solvent, do not reach the brain and do not show any medicinal effects, unless a patented technology is used to increase the brain mobility of diosgenin ( (*RESILIO COMPANY LIMITED) No effect on cognitive function is expected. In a clinical study conducted by us in healthy subjects, a test product based on this patented technology was used, and as a result, the effect on cognitive function was observed. Based on the scientific evidence and the patent, Diosgenin Gold was accepted as a functional food product with the indication of “maintaining cognitive function, which declines with age, in healthy middle-aged and elderly people. It should be noted that functional food product notifications related to cognitive function usually have to be limited to “support (maintain) short-term memory, which is a part of cognitive function (e.g.),” but the functional food product notification accepted by RESILIO appears to cover the overall cognitive function.
References.
1) Alzheimer’s Association: Alzheimer’s & Dementia, 15, 321-387 (2019)
2) Villemagne VL. et al.: Lancet Neurol, 12, 357-367 (2013)
3) Reiman EM. et al.: Lancet Neurol, 11, 1048-1056 (2012)
4) Jack CR Jr. et al.: Brain, 132, 1355-1365 (2009)
5) Bateman RJ. Et al.: N Engl J Med, 367, 795-804 (2012)
6) Gordon BA. Et al.: Lancet Neurol, 17, 241-250 (2018)
7) Ward A. et al.: Dement Geriatr Cogn Dis Extra. 3(1), 320-332 (2013)
8) Honig LS. et al.: N Engl J Med. 378(4), 321-330 (2018
9) Egan MF. et al.: N Engl J Med. 378(18), 1691-1703 (2018)
10) Gauthier S. et al.: , Lancet. 388(10062), 2873-2884 (2016)
11) Tohda C. et al.: Sci Rep. 2, 535 (2012)
12) Yang X. et al.: Sci Rep, 8, 11707 (2018)
13) Tohda C. et al.: Nutrients, 9, E1160 (2017)
14) Tohda C. et al.: Sci Rep, 3, 3395 (2013)
15) Lomenick B. et al.: Proc Natl Acad Sci USA, 106, 21984-21989 (2009)
16) Yang X. et al.: Front Pharmacol, 9, 48 (2018)
Figure 1: Mechanism of action of diosgenin in neurons
Diosgenin directly stimulates 1,25D3-MARRS and activates at least PI3K, ERK, PKC and PKA. 1,25D3-MARRS activation leads to enhanced memory. In addition, diosgenin leads to a decrease in HSC70 expression in neurons; the axonal atrophy induced by Amyloid β is reduced by the reduction of HSC70 and normal axonal growth is promoted.
Figure 2: Changes in cognitive function after 12 weeks of administration of yam extract.
Yam extract containing high concentrations of diosgenin was encapsulated and administered to healthy subjects for 3 months. The placebo group was used as the control group. The groups were assigned in a randomized, double-blind manner; the total RBANS score and the five index scores that made up the total score were compared when subjects were divided into those younger than 60 years of age or older. There was a significant improvement in total RBANS scores and delayed memory after taking yam extract (modified from Reference 13). (two-tailed paired t-test, n = 28)